Abstract
Introduction
Autoimmune cytopenias (AIC) are prevalent in patients with primary immune deficiencies, including common variable immunodeficiency (CVID). They are often refractory and difficult to treat, and patients with autoimmune complications of CVID have a higher risk of mortality than those with an infectious phenotype (Farmer et al. Front Immnol 2017; Resnick et al. Blood 2021). The pathogenesis of AIC in CVID is not well understood, making diagnosis and prognostication challenging. We previously observed a block in development from transitional to follicular B cells in patients with activated PI3K delta syndrome (APDS), a monogenic form of CVID (Farmer et al. Sci Signal 2019). We have now investigated a larger cohort with various causes of monogenic CVID (45% with AIC). We sought to determine if a block in B cell development extended beyond APDS to other patients with monogenic CVID and autoimmunity. We aimed to identify a link between the transitional B cell block and a break in tolerance, as transitional B cells are immature cells with self-reactive properties prior to differentiating into mature follicular B cells (Allman et al. Curr Opin Immunol 2008). Thus, we performed flow cytometry using the 9G4 monoclonal antibody to identify B cells containing the 9G4 idiotope of the immunoglobulin heavy chain VH4-34 gene. This idiotope is associated with autoimmunity (Cappione et al. J Clin Invest 2005; Pugh-Bernard et al. J Clin Invest 2001). Utilizing single-cell Cellular Indexing of Transcriptomes and Epitopes by Sequencing (scCITE-Seq) and B cell receptor (BCR) repertoire, we analyzed patients with APDS to further define heterogeneous B cell populations. We leveraged our access to biospecimens and clinical data from patients with monogenic CVID to study pathologic B cells.
Methods
Patients were recruited in accordance with the Partners Institutional Review Board. The cohort was queried for cytopenias, including immune thrombocytopenia (ITP), autoimmune hemolytic anemia (AIHA), and autoimmune neutropenia (AIN), identified by positive history on physician chart review or otherwise captured by review of all clinical details in the Electronic Medical Record. We performed flow cytometry on peripheral blood mononuclear cells with a detailed B cell panel including the 9G4 antibody. We included 20 patients with monogenic CVID (PI3K (n=4), NFKB1 (n=5), and CTLA4 mutations (n=11)) and 12 healthy controls. Nine patients had AIC (ITP (n=3), ITP + AIHA (n=4), ITP + AIN (n=1), ITP + AIHA + AIN (n=1)). We sequenced total CD19+ B cells from 4 patients with APDS and 4 controls using scCITE-Seq. With the Immcantation software suite, we identified clonal clusters based on BCR sequences and constructed maximum parsimony lineage trees for clusters with >5 unique sequences.
Results
Patients with monogenic CVID and AIC had marked expansion of 9G4+ B cells compared to healthy controls. There was a statistically significant (p<0.05) increase in the percent of 9G4+ B cells in patients with AIC compared to controls in the transitional T1/2, T3a, and T3b B cell subsets, as well as double negative, follicular, activated naïve, marginal zone precursor, and marginal zone subsets. This expansion was particularly pronounced in the transitional B cell population (p<0.001).
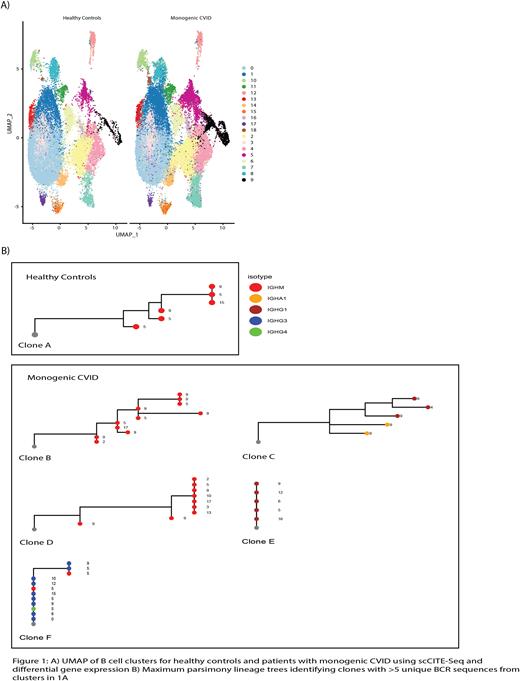
ScCITE-Seq identified 19 B cell clusters. Clonal B cell populations separated into three predominant IgM isotype clusters in healthy controls (5, 9, and 15), while in patients with APDS there were additional clusters identified, including IgM isotype clusters 0, 2, 3, 10, 13, 17 and IgG isotype clusters 4, 6, 10, 12, and 16 (Figure 1). This suggests that there are novel, isotype-switched B cell states present in patients with APDS that are not present in healthy controls.
Conclusions
In this study, we investigated B cell populations in patients with monogenic CVID and autoimmunity. We found expansion of transitional B cells characterized by 9G4 positivity. These transitional cells may fail to be eliminated because they are aberrantly activated and differentiate into self-reactive activated B cells and plasmablasts that secrete pathogenic autoantibodies. ScCITE-Seq revealed novel B cell subsets in patients with APDS and could represent an altered state of B cell biology that leads to pathologic autoantibody production and clinical autoimmunity. These results improve identification of heterogeneous B cell subsets and connectivity between subsets.
Disclosures
Allard-Chamard:Pfizer: Membership on an entity's Board of Directors or advisory committees, Other: Clinical trial, invited talk; Neomed: Other: Clinical trial; Daiichi Sankyo Inc: Other: Clinical trial; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: Clinical trial, invited talk; Sanofi: Other: Clinical trial; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: clinical trial; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Other: Clinical trial, invited talk; DIEX: Other: Clinical trial; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: invited talk; Celltrion: Membership on an entity's Board of Directors or advisory committees, Other: invited talk; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: invited talk; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Other: invited talk; Mantra Pharma: Other: invited talk; Sobi: Other: invited talk; Sandoz: Other: invited talk. Farmer:Bristol Myers Squibb: Research Funding; Pfizer: Research Funding. Pillai:Abpro: Membership on an entity's Board of Directors or advisory committees; BeBio: Membership on an entity's Board of Directors or advisory committees; Paratus: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal